MICHAEL HARDCASTLE
Splenectomy for nodular splenic masses or diffuse splenomegaly is common, and we often receive entire spleens, or parts of splenic lesions for histopathology. Some spleens (and splenic masses) are very large, so fixing adequately in formalin prior to submitting to the laboratory for histopathology can be problematic.
The entire spleen is our preferred sample, however some spleens or splenic masses are very large and difficult to fix in adequate formalin before submitting them to the laboratory. For this reason veterinarians often need to take sub-samples from splenic lesions.
Unfortunately, the diagnostic area of a splenic mass can be missed with sub-optimal sampling. The following is a list of tips for optimising splenic lesion sampling.
> The bulk of the mass will probably be a non-diagnostic haematoma, therefore the centre of the mass is not usually worth sampling (Figure 1).
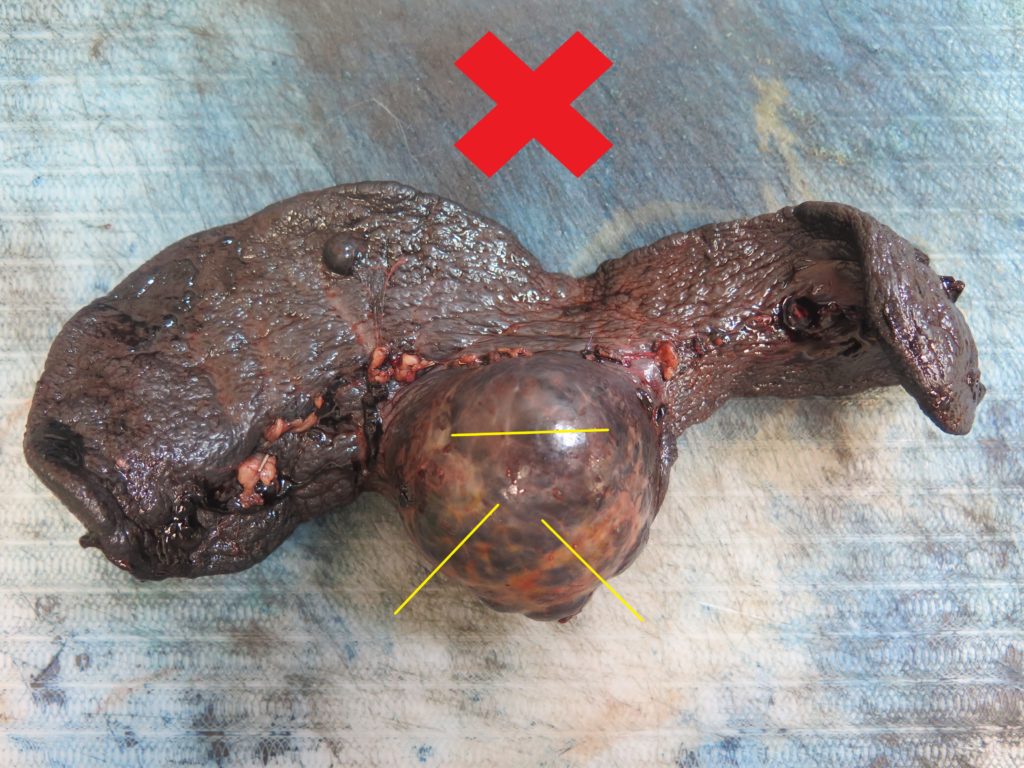
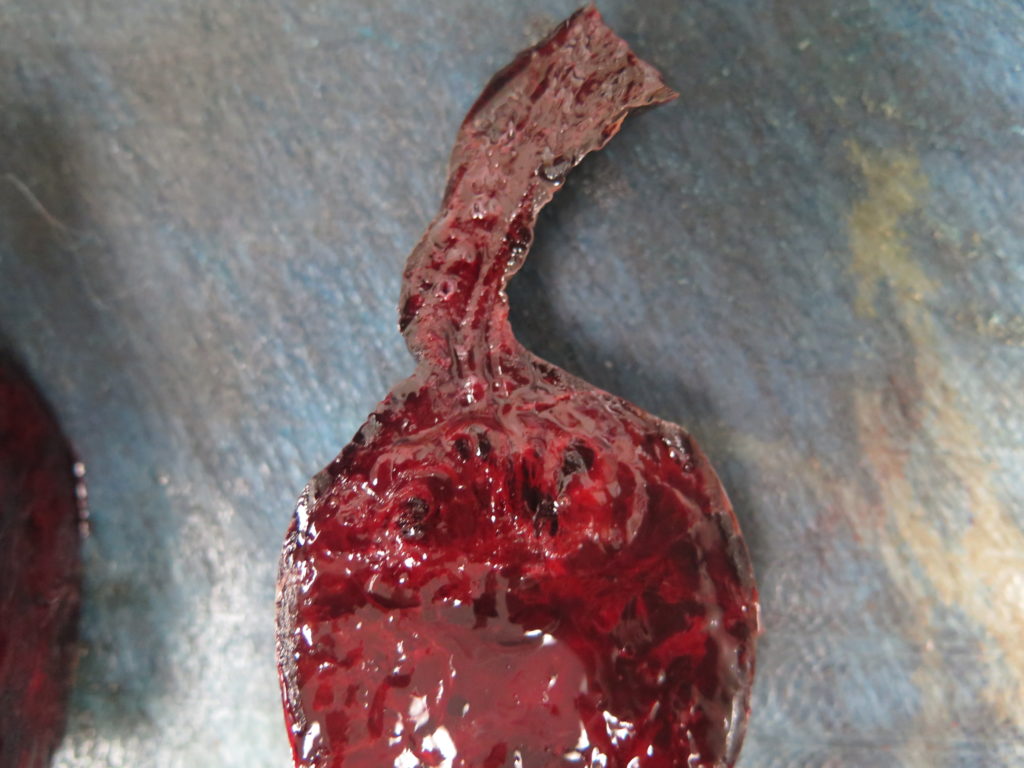
> Ideally, collect 4-6 samples from the mass/spleen interface (Figures 2, 3), since this tends to be the region where any neoplastic cells are best preserved and most plentiful.

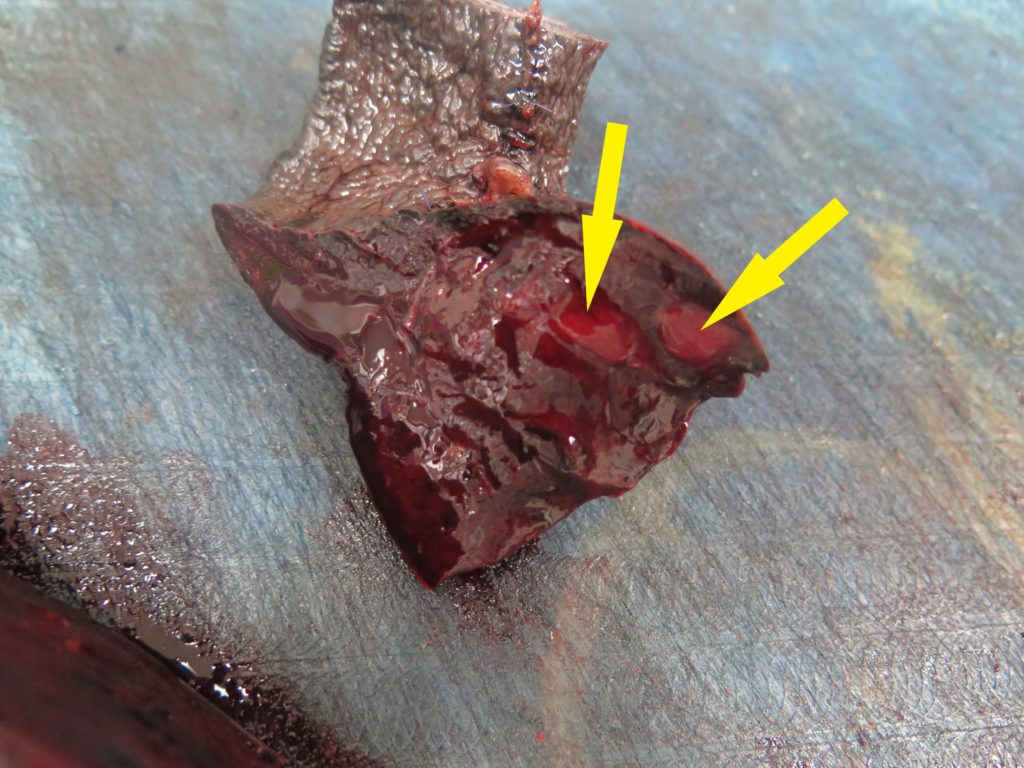
> It is worth also “bread-loafing” the mass to look for any discrete areas of differing texture, colour etc. that might indicate an additional lesion, and also sampling those (Figure 4). White or tan areas in particular should be collected.
> Any additional masses should also be sampled (Figures 2, 5). It is not uncommon for haemangiosarcoma to present as multiple splenic masses, or alternatively, for there to be several different lesions within the same spleen. All masses should be sampled, unless they are myriad – in which case a selection will need to be made.

> Diffusely enlarged spleens can usually be sampled adequately by collecting wedges from several random locations, however it also would be worth “bread-loafing” the spleen to check for any grossly different areas to sample as above.
> Collect wedges of 1-2 cm thickness in order to allow for rapid fixation while also minimising warping of the tissue during fixation.
> Finally, it is worth keeping the rest of the spleen in the freezer at your clinic while awaiting the histopathology results; it is likely to be of low diagnostic value due to freezing artefact, but it could be a “last resort” back-up if initial sub-samples are not diagnostic.

