Kathryn Jenkins
Lymph nodes are commonly sampled for cytologic evaluation. Cytology can provide a rapid, non-invasive and cost-effective diagnosis in many disease processes including lymphoma, metastatic disease, lymphadenitis and reactive lymphoid hyperplasia. Although lymph nodes can be easy to sample, it is often a challenge to generate diagnostic quality cytology smears. These require a thin, highly cellular preparation of intact cells, from representative locations.
The following are some tips and techniques that can help increase the diagnostic power of lymph node aspirates for cytology, helping avoid a non-diagnostic result. Many of these tips can also be applied to cytology in general.
Sample technique
· Non-aspiration method
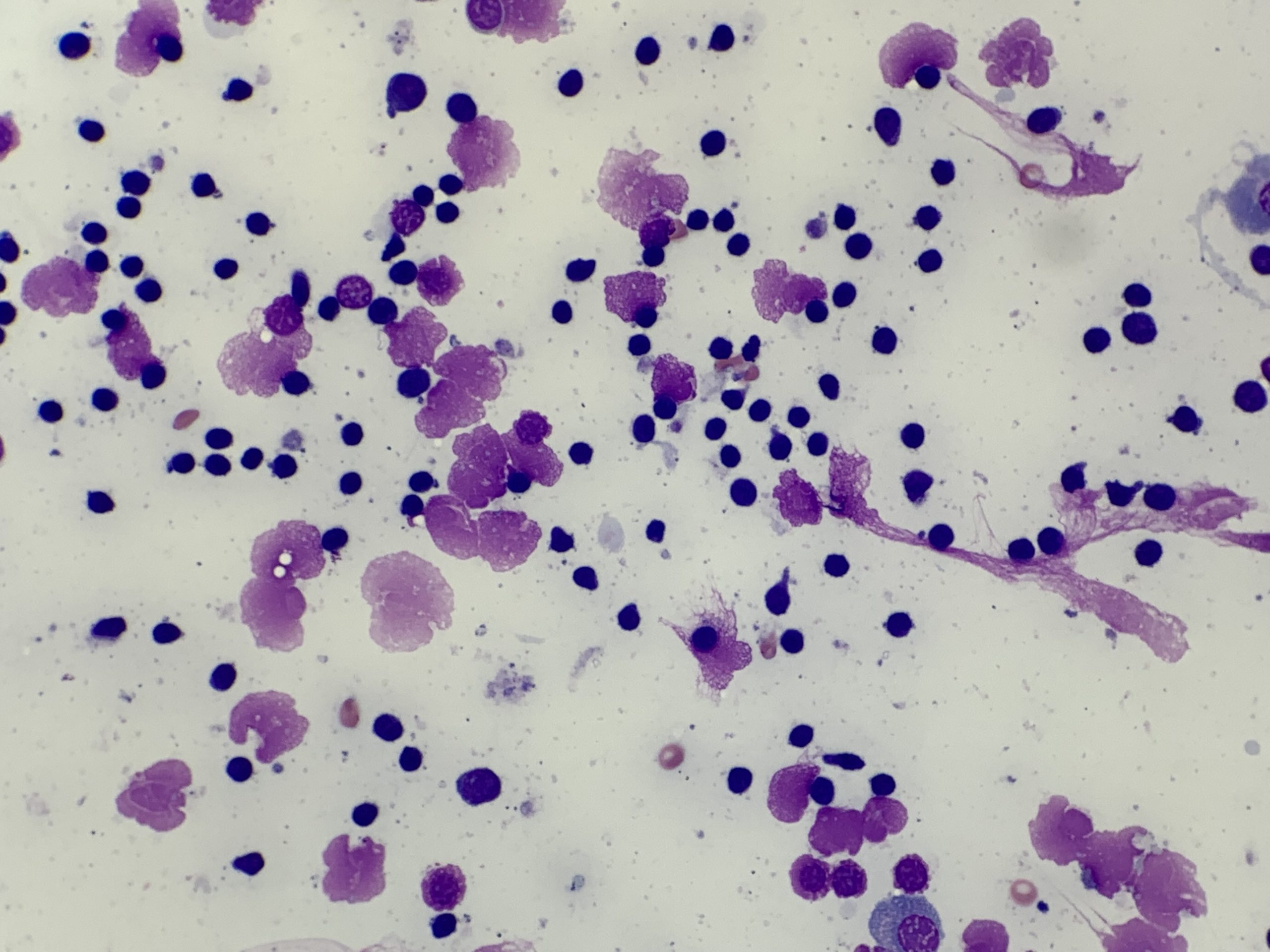
Intact cells are critical to be able to evaluate both cell size and morphology. This can be especially challenging when sampling lymph nodes, due to the inherent fragility of lymphocytes. A non-aspiration technique (woodpecker or needle-only) can help maintain cell preservation, and lymphoid tissue generally exfoliates well without the need for the increased pressure of aspiration. Note that ruptured cells should not be evaluated, as they often appear bigger, and can show apparent prominent nucleoli, which may lead to a potential misdiagnosis.
For the non-aspiration method a 21-23 gauge needle is recommended. In addition, attaching a pre-filled 3-5 mL syringe for this method allows for additional stabilisation, as well as immediate expulsion of the material onto a glass slide. [Note: if using an aspirational technique, pressure must be released from the syringe prior to removal from the tissue, and then the syringe must be detached and re-filled with air to expulse the material]. The non-aspiration method uses capillary action and needle re-direction to collect tissue and usually requires only 3-5 redirections (without removal from the lymph node) to gather sufficient material.
· Blood
A second tip is that blood contamination can be useful. The plasma present in blood can help preserve cellular morphology (Figure 1B). However, very thick blood on cytology tends to obscure cell detail and should be avoided. Reducing redirection attempts and sampling different areas of the node or mass is recommended to help avoid this.
· Location
It is important to generate representative slides for cytologic evaluation, as this increases the diagnostic power of cytology. In cases of generalised lymphadenopathy sample several lymph nodes, ideally two smears each from 3-4 enlarged lymph nodes. When only a single lymph node appears enlarged, sample 3-4 different areas within the lymph node. This is especially true with large or rapidly growing lesions, as the centre may contain non-diagnostic necrotic or haemorrhagic material.
Note that submitting cytology from multiple lymph node locations generates a single cytology fee when submitting to Awanui Veterinary (see our current price book for further details).
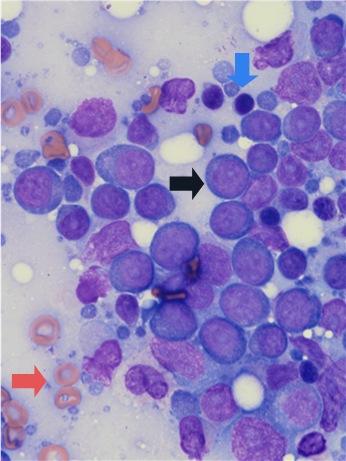
Sampling too few locations can increase the potential for diagnostic errors. Examples include a misdiagnosis of lymphoma when lymphocytes are aspirated from germinal centres in reactive lymph nodes (Figure 2), or missing a diagnosis of metastatic disease when a lymph node is not yet fully effaced by the tumour.
· Special considerations for the submandibular area
Enlargement of submandibular lymph nodes is easily detected and sampled. However, submandibular lymph nodes are constantly exposed to antigens and reactive hyperplasia can complicate cytologic interpretation at this location. In addition, salivary glands also reside in close proximity to the submandibular lymph nodes, and may be inadvertently sampled. For these reasons in cases of generalised lymphadenopathy, it is important to include sampling the popliteal and prescapular lymph nodes alongside the submandibular lymph nodes.
Smear preparation
Expulsing the tissue on to a glass slide and smearing the material must be performed immediately after sampling, to avoid the tissue drying out.
There are several reported techniques for creating a cytology smear. For lymph node aspirates the gentle glass-on-glass smear technique is considered the most effective, both to maximise cell integrity and generate a thin monolayer of tissue for evaluation (Figure 1, A). However, you may want to utilise different techniques for the same case (such as the roll technique, or impression smears described under Cytology preparation techniques), to help increase the chances of obtaining a diagnostic sample.
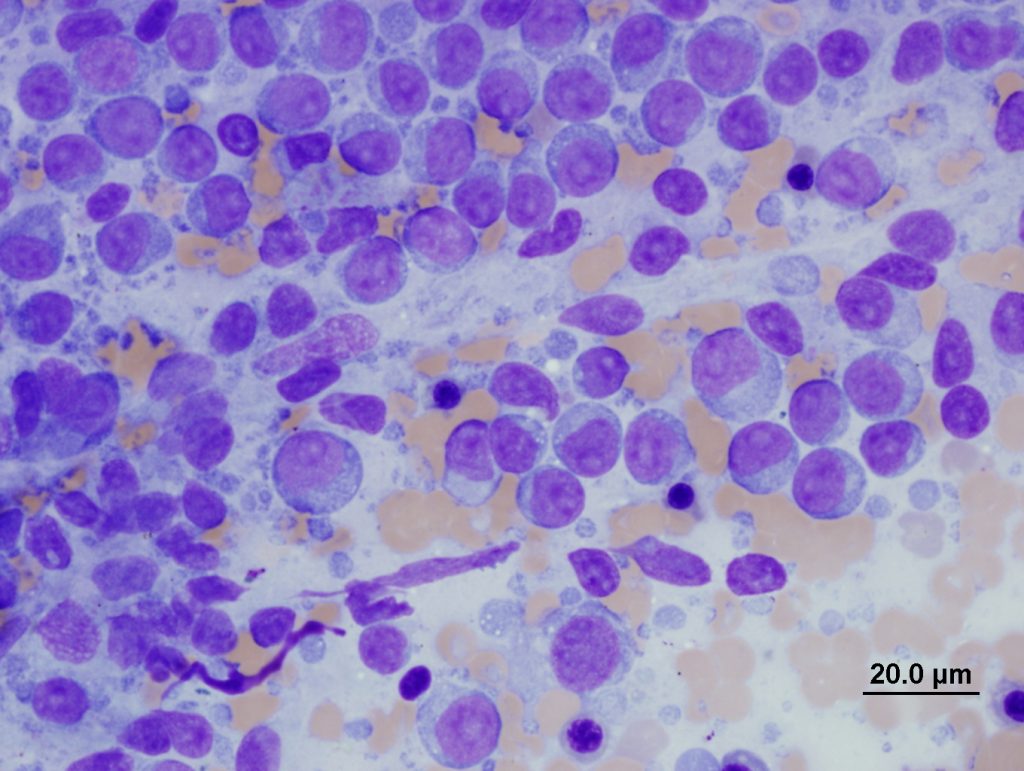
Other methods (including splatter, squash, scribble or starfish techniques, see Figure 3.) often produce suboptimal quality samples, generating both thick areas of tissue (which causes cell contraction and under-staining), and excessive rupture of cells (Figure 4, C-D). Assessing lymphocyte size is a crucial part of interpretation, and contracted cells in thick areas can appear small, and ruptured cells can appear large which may result in inaccurate interpretation.
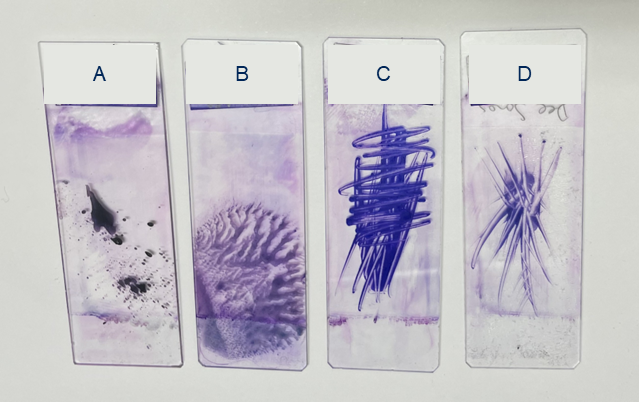
Figure 3. Examples of suboptimal sample preparation techniques – These techniques should be avoided as they create areas of thick and ruptured tissue, which limit cytologic evaluation. A: Splatter B: Squash C: Scribble D: Starfish
Note that when ultrasound guidance is used, the gel must be wiped from the skin surface prior to sampling for cytology, as even small amounts can reduce stain quality and obstruct viewing of tissue cells (Figure 4, A). Clearly label slides in pencil (as pen or markers can dissolve during staining).
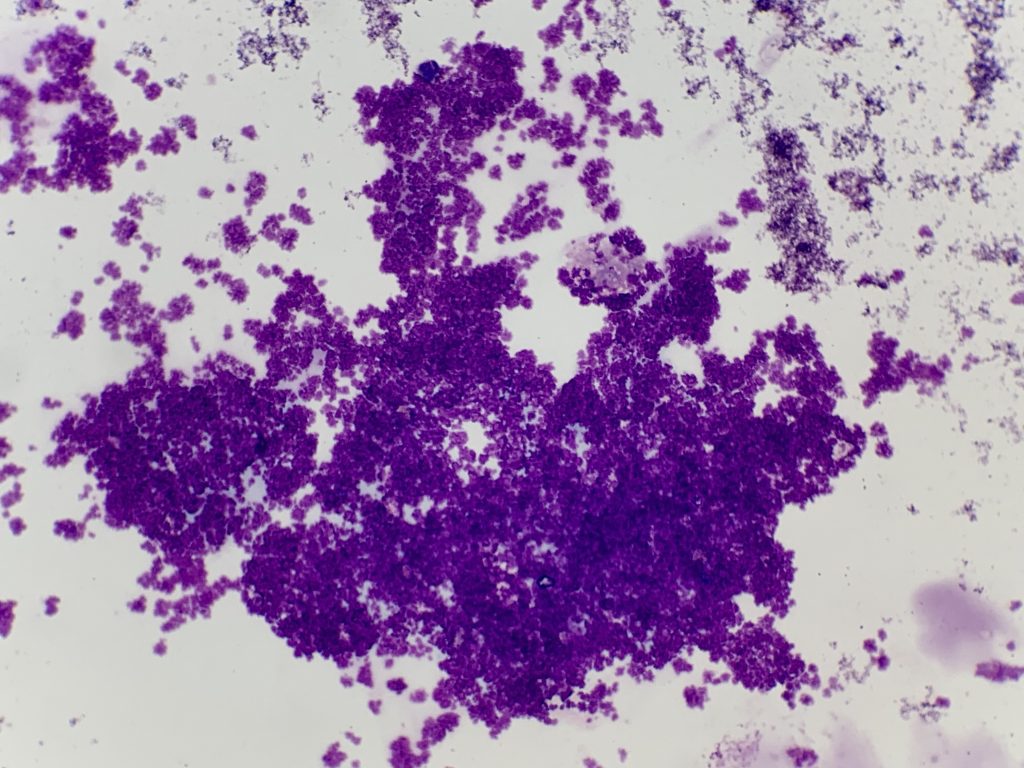
Ultrasound lubricant gel (purple granular material).
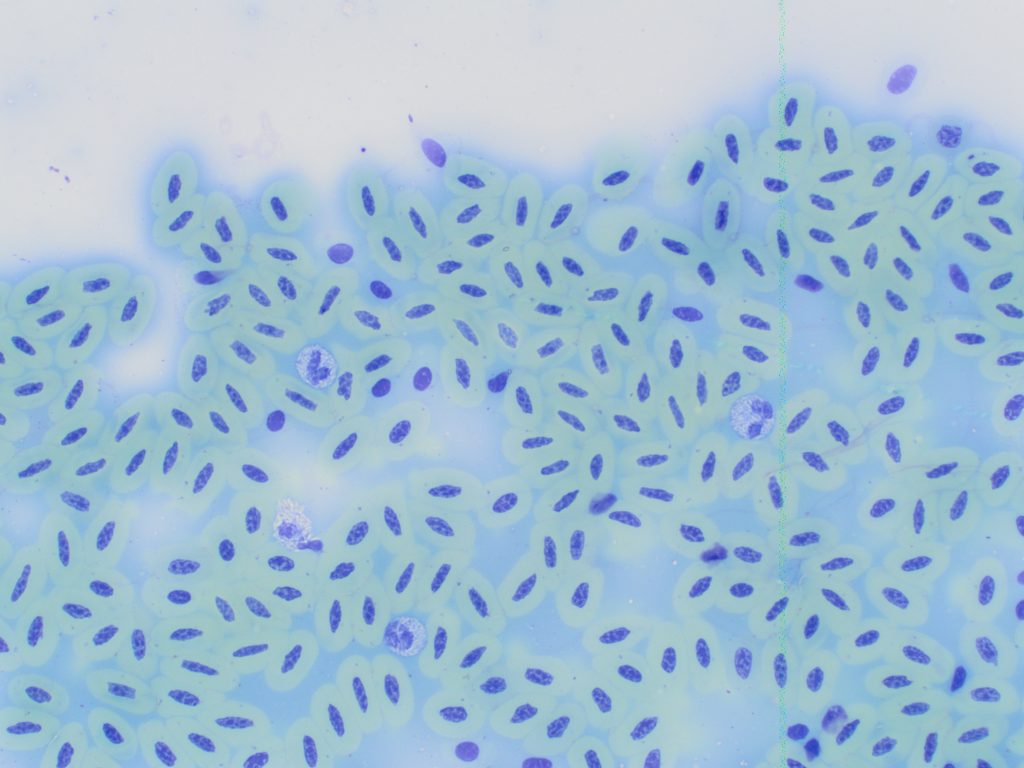
B: Formalin fixation (green staining erythrocytes).
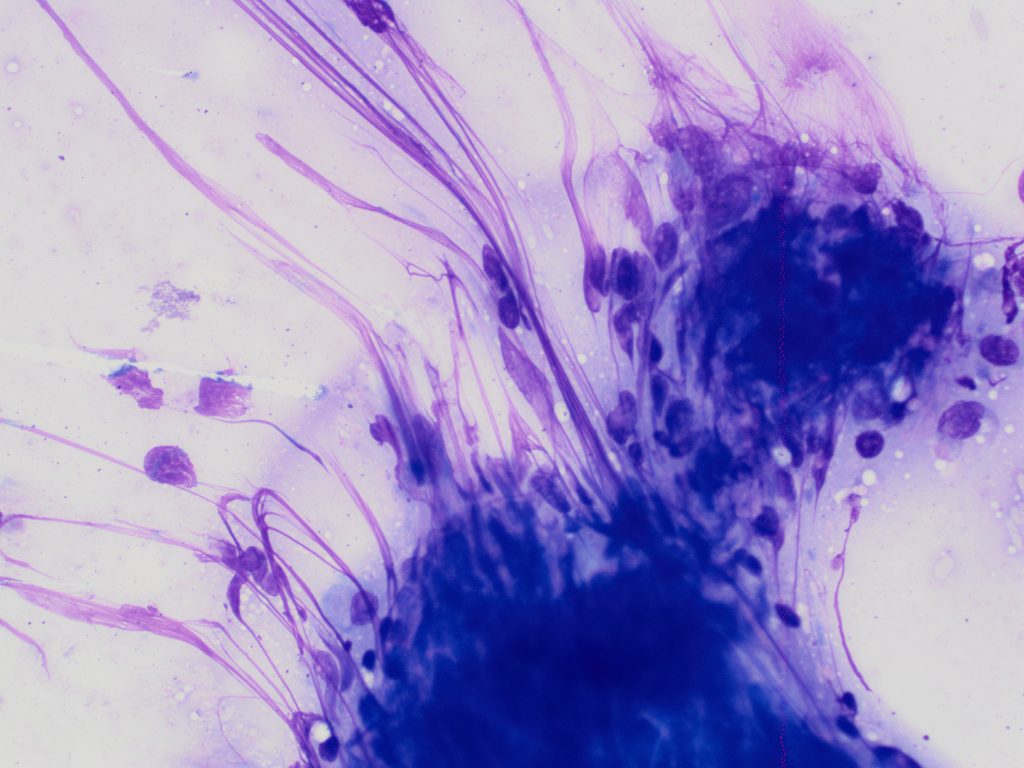
C: Thick smear with cell rupture (nuclear streaming).
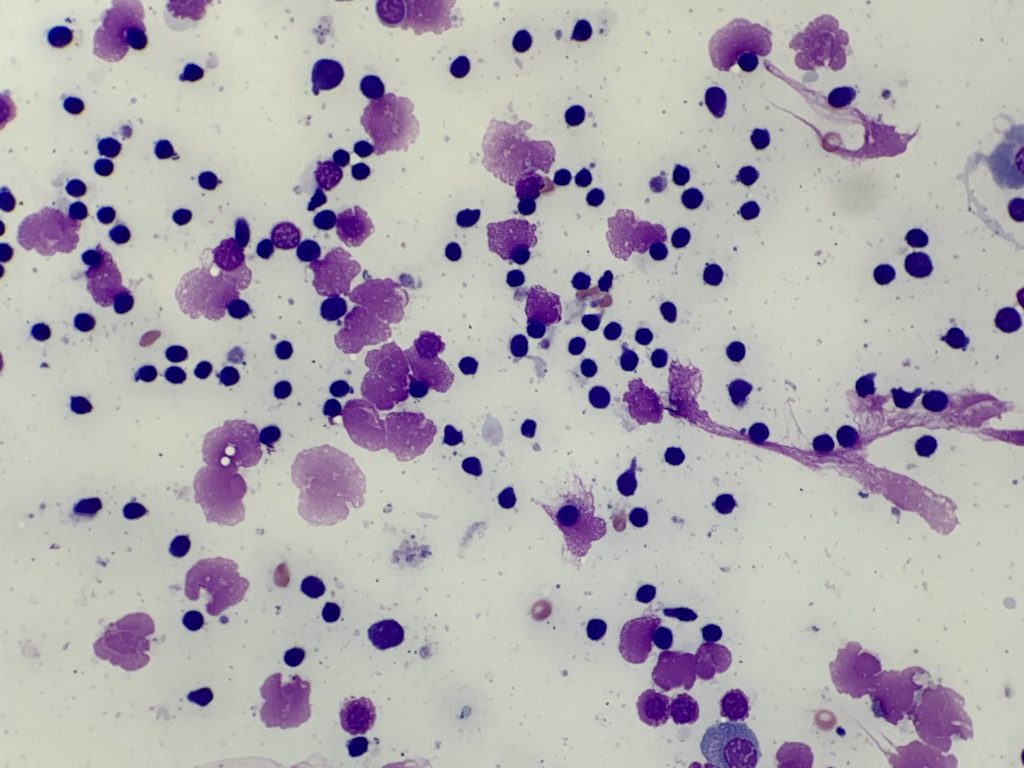
D: Squash preparation with dark contracted lymphocytes and pale ruptured lymphocytes.
Regardless of the technique used, grossly a successful lymph node cytology sample appears fluidic and creamy white, indicating the presence of many tissue cells. Allow to air dry completely before staining. In colder climates, cytology samples can be dried rapidly with a hair dryer (on low setting) to avoid slow drying artifacts which reduce cell preservation. This also works well for joint cytology samples.
Sample staining and submission
Whether cytology slides are to be evaluated in-house or sent to a reference lab for pathologist review, ideally at least one representative slide should be stained and evaluated in-clinic, to assess the diagnostic quality of the sample (i.e. check if there is adequate cellularity and preservation to enable an interpretation). This increases sensitivity and maximises the diagnostic power of cytology. Doing this before the patient goes home allows time for a repeat sample if required.
Follow your routine staining procedure for in-house evaluation. Performing a quick scan on low power after initial staining can identify whether the sample needs more time in the stains (which can be required with thicker lymph node preparations). Poorly stained smears often look pale and pink (rather than the purple and blues of nuclei and cytoplasm respectively).
Note also that quick stains (such as Diff-Quik) can make lymphocyte chromatin appear coarse and nucleoli more prominent, compared with alcohol-based stains (such as modified Wright stains). The latter are used in both the reference laboratory and are also commonly seen in the images used in many reference materials.
Remember to use a cover slip when using the 40x (dry) objective, otherwise the cells will appear fuzzy. A drop of oil can then be placed on top of the coverslip when rotating around to the 100x (oil) objective.
If sending the samples to a reference lab be sure to also include the pre-stained slides (as these may be the most diagnostic samples) and remove any coverslips (as these can stick to the glass as the oil dries out). Also include the signalment, relevant history and clinical description, as these form a critical component of the interpretation.
Useful references:
· Meichner K. Cytology of Lymph Nodes. Today’s Veterinary Practice. Issue: May/June 2023.
· Raskin R. and Meyer D. Canine and Feline Cytology. Third Edition. Elsevier. 2016.
· Schlemmer S. Obtaining a Sample for Cytology Using Fine Needle Biopsy. Today’s Veterinary Practice. Issue: January/February 2023.
· Sharkey L. C. Veterinary Cytology. John Wiley & Sons, Inc. 2021.