Things to consider when choosing a point of care (POC) analyser
1. Clearly define your requirements
As an initial starting point, it can be very helpful to set out your expectations and requirements for an analyser before you even consider the devices on offer. A vast amount of information and marketing pressure can be thrown at you from companies and it is easy to lose sight of what your individual requirements are and what features are most important to you. These requirements are unique to your clinic, the clients you service and your preferences as a veterinarian.
Some key questions to answer are:
· What type of testing are you most likely to perform?
> e.g. pre-anaesthetic checks, wellness screens, out-of-hours/emergency samples, sick animal samples.
· What tests do you wish to have available?
> Make a list of must-haves and nice-to-haves.
· Who will be operating the analyser and at what level do you think you will able to operate at?
> Think about the level of training and capabilities of your staff, staff turn-over, time available for staff to operate and monitor the analyser. This will help you to determine how important usability and the simplicity of the analyser will be in the decision-making process. Additionally, some testing such as haematology, requires a high degree of knowledge to monitor and validate results. If it will be difficult to fulfil these requirements other options may be worth considering e.g. manual PCVs and use of an external reference laboratory.
· How many samples do you predict you will run per week?
> If the numbers per week for a particular test is likely to be low (e.g. < 5/week) and it is not time-sensitive, it may be more cost-effective and of higher quality to use a reference laboratory. Predicting test numbers is also useful for working out the costs associated with an analyser and how it compares to other analysers.
· What is your work flow for performing laboratory diagnostics?
> Again, consider how many samples you are likely to run at one time, when these samples will be collected, when are results needed and how that will fit in with the rest of the work being completed in the clinic. Different analysers vary in their analysis time, complexity of use and steps that need to be performed prior to testing (e.g. reagent warming time, loading and maintenance requirements etc.) and this may be of greater or lesser importance depending on testing volumes, clinic work-flow and staffing.
· Where will the analyser be situated? Are there any space considerations? Do you require Ethernet/ internet connection and therefore cabling?
> If you are considering analysers for a multi-clinic situation or are a large multi-vet practice, it may be a valuable exercise to perform a survey/create a questionnaire to gather feedback from your staff/key users and to characterise what is currently occurring in each clinic.
2. Rank the importance of the analyser characteristics
Hopefully the above will have crystallised what role you are expecting an analyser to fulfil and how it will sit within your working environment. Every analyser has its pluses and minuses and thus in addition to listing your requirements it can also be useful to rank what is most important to you so that you can determine what you are willing to compromise on and ultimately which analyser will be the best fit for your practice.
The following are some features to consider and rank:
· Test selection – what tests and panels are available? Do the panels have the analytes you want on them?· Test flexibility – is it important for you to be able run single tests or will the panels suit most of your testing situations?
· Sample volume
· Analysis time – this may be important if a large number of samples are needed to be run at a particular time of day or depending on clinic work flow.
· Analyser size and any associated equipment – printers, centrifuges, integration/internet cabling, fridge space etc.
· Usability of the analyser and complexity of operation, maintenance requirements etc.
· Analytical performance (further guidance is provided below)
· Customer support – what are expectations when faults occur, do they have an efficient service for providing technical assistance, other user resources etc.
· Reporting/integration – can you connect the analyser with your clinic/patient database, can you generate client reports etc.
· Availability of quality control material and functionality on analyser – helpful if you wish to perform testing to a gold standard and may help mitigate inexperience of users or less robust quality monitoring.
· Cost – dependent on number of tests likely to be run, cost of consumables, open shelf-life of reagents, maintenance requirements etc.
· Fellow veterinarian feedback – talking to other vets about how they have found certain analysers can be useful for assessing the usability of the analyser and the quality of customer service/technical support provided by companies. It can also be a useful way to gather feedback about reliability but be aware that prior conceptions about an analyser can significantly colour a veterinarian’s perception about its reliability and in particular comments about analyser accuracy and performance are very rarely based on objective data. Asking about specific aspects or details of issues may help you to assess the quality of the feedback.

3. Determine analytical performance
Analytical performance is important as ultimately if an analyser produces incorrect results, it really doesn’t matter what other characteristics it has (timeliness of results, test selection, usability, cost etc.). It is important to realise that there is no registration or accreditation requirements for an analyser to be marketed for veterinary use and it is up to the end user to satisfy themselves that an analyser is fit for purpose. Having knowledge of the operating performance of your analyser also makes you aware of its limitations and if any particular tests need to be treated more cautiously or with closer monitoring/checking. This knowledge can also be used for determining what quality control procedures are needed to monitor an analyser.
Analytical performance can be broken down into two key characteristics: accuracy and precision
> Accuracy is a measure of how close the analyser’s results are to the ‘true value’ and is often denoted as the amount of ‘bias’ an analyser shows. In comparison studies, accuracy is often assessed by comparing an analyser with a commercial analyser in a reference laboratory. The commercial analyser’s results are taken as being representative of the ‘true value’ but in reality the analysers often use different test methodologies and therefore it is not unexpected for them to produce different results. Results from participating in an External Quality Assurance programme/Proficiency testing programme is another way that an analyser’s bias can be assessed. In this case an analyser’s results can be compared with other analysers of the same type, providing a more level playing field and fairer assessment of bias. Finally if the manufacturer is able to supply quality control material, which has target values for your type of analyser, bias can be assessed and monitored using this material.
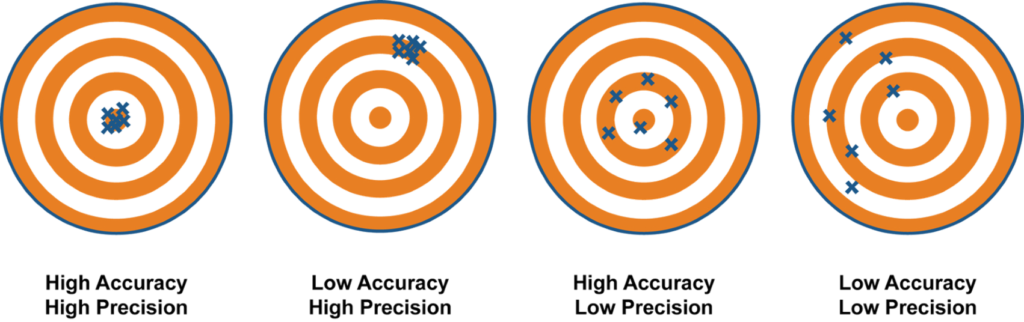
> Precision is a measure of how repeatable a measurement is. An analyser can produce results that on average are close to the true value but that have a lot of random variation around that mean and vice versa an analyser can be very precise but the results are not close to the true value. The picture below illustrates the difference between these two analytical characteristics.
Precision is usually determined by repeatedly running a patient sample or quality control material over a day or a number of days. The standard deviation of the results is a measure of the precision of the analyser although results are often reported as coefficient of variations or CV%. This is basically the standard deviation converted into a percentage of the mean so that comparisons can be made across different tests and is not concentration or unit of measurement dependent.
Once the bias and the CV% has been determined, the total observable error for each test on an analyser can be calculated. This is a measure of the overall amount of error that an analyser could have present when it runs a particular test. The total observable error can be compared against guidelines that have been set for the total allowable error (TEa) that is acceptable for veterinary samples.1 If the total observable error for a test on the analyser is greater that the TEa guideline, it indicates that the results the analyser produces could on some occasions be far enough away from the true value that errors in clinical decision-making may occur. If the total observable error is below the TEa guideline, the degree of error produced by the analyser is generally not large enough to result in errors in clinical judgement.
Where can you find information about the analytical performance of an analyser?
In some cases there may be published studies for a given analyser in the scientific literature. The manufacturer will usually also have internal data of their own performance assessments on the analyser. Sometimes the data presented however may be limited to regression analysis and correlation coefficients. These values give an indication of whether the analyser’s results respond similarly to a reference analyser but do not assess precision and may not fully characterise the degree of bias present.
A further point of note for both published studies and manufacturer supplied information, is that the data may only be from the assessment of one single analyser. While it is hoped that analysers of the same type would perform similarly, studies have indicated that analytical performance can vary significantly between individual analysers even of the same make and model.2 It is therefore worth considering performing your own performance assessments when you trial or buy a new analyser.
Finally, analysers may often vary in their performance across different tests, performing well for some tests and less well for others. It is useful when reviewing performance data to again keep in mind which tests are of most importance to you.
References:
1. Harr KE, Flatland B, Nabity M, Freeman FP. ASVCP guidelines: Allowable total error guidelines for biochemistry. Veterinary Clinical Pathology. 42:424-436, 2013.
2. Rishniw M, Pion PD, Maher T. The quality of veterinary in-clinic and reference laboratory biochemical testing. Veterinary Clinical Pathology. 41:92-109, 2012.

