SANDY WELTAN
There are many advantages to performing cytology. It is generally quick and simple to perform with rapid results, inexpensive and minimally invasive.
Inconclusive results from non-diagnostic slides are a source of frustration for the practitioner and the pathologist. A good slide contains an adequate number of intact, appropriately stained cells in a monolayer which is representative of the lesion sampled.
Starting at sampling, only clean, dry, new slides should be used. The skin can be moistened with alcohol, but gels should be avoided. Depending on the tissue, needles should be 21-23g and syringes 3-10mL. Whether to use suction or not depends on the tissue aspirated. Suction is best avoided on soft or vascular tissue. The best place to aspirate a mass is close to the margin, avoiding the necrotic centre.
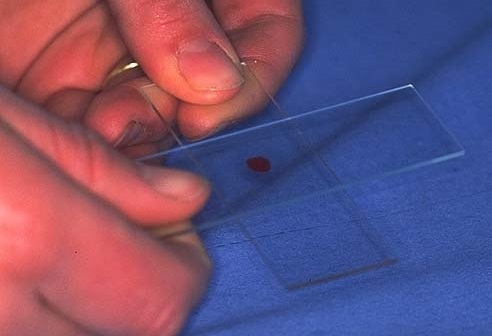
Most cell damage occurs with transfer from the needle to the slide. Hold the needle close to the slide and gently expel a single drop 1-2 mm in diameter which is spread with a gentle slide on slide technique (Figure 1).
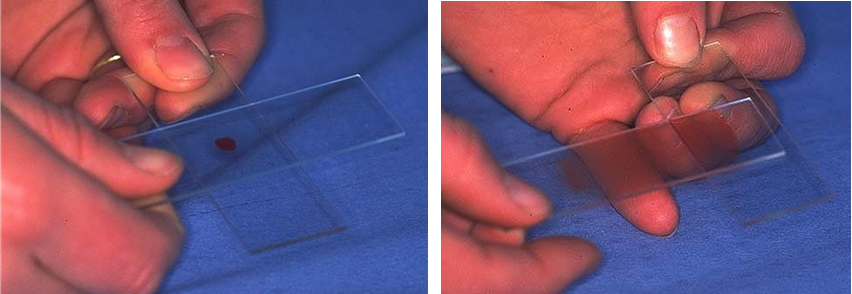

For examples of smears that result in poor cell preservation see Figure 2: left – too thick; middle – ruptured cells; right – areas too thick and ruptured cells. Smears that give good cell preservation can be found in Figure 3: top – blood smear technique, good for fluid samples; middle – see technique in Figure 1, works for most tissues; bottom – for fragile cells.

Artefacts can also result in samples that cannot be interpreted. These include exposure to heat or cold, moist slides, exposure to formalin fumes, ultrasound or other gels or ointments and keratin contamination from touching the slide surface.
For sampling mucosal surfaces and nasal cavity, a cytology brush is very useful as it is abrasive enough to sample deeper than the mucosal surface. The cytology brush is then rolled onto the slide. Note: Cytology brushes will be available via our online store in the near future.
Cytology can also be performed on fluids. Please submit in EDTA or plain serum tubes (clot activating tubes are not recommended as they contain silicon, which coats cells and disrupts their morphology). Please take note of special sampling conditions for CSF which can be found in the Vet Handbook on our website here. There you will also find helpful tips for collecting other samples.